Laws of Chemical Combinations: Understanding the Basics
Chemical combinations, or chemical reactions, occur when elements come together to form compounds. These reactions follow specific rules, ensuring predictable outcomes. Let's break down the five fundamental laws governing chemical combinations:
1. Law of Conservation of Mass
- Chemical reactions follow predictable rules.
- Elements combine in specific ratios.
- Mass remains constant during reactions.
Simplifying Complex Chemistry
Understanding these laws provides a solid foundation for exploring chemistry. By recognizing patterns and relationships, you'll better grasp chemical reactions and compound formation.
- Matter cannot be created or destroyed, only transformed.
- Total mass before reaction = Total mass after reaction.
Example: Burning wood (mass remains constant, only form changes)
2. Law of Definite Proportions
- Compounds always contain elements in fixed ratio.
- Composition remains constant, regardless of source.
Example: Water (H2O) always has 2 hydrogen atoms for every 1 oxygen atom.
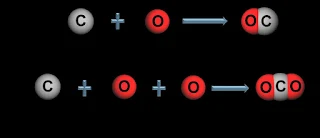
3. Law of Multiple Proportions-
- Elements combine in simple whole-number ratios.
- Different compounds formed from same elements have different ratios.
Example: Carbon and Oxygen form CO (1:1) and CO2 (1:2)
4. Law of Reciprocal Proportions
- When two elements form compounds with third element, ratios of first two elements remain constant.
Example: Sodium (Na) and Potassium (K) compounds with Chlorine (Cl) have consistent Na:K ratio.
5. Law of Combining Volumes (Gay-Lussac's Law)
- Gases react in simple whole-number volume ratios.
- Volumes of reacting gases related to volumes of products.
Example: 2 volumes Hydrogen (H2) + 1 volume Oxygen (O2) → 2 volumes Water (H2O)
Key Takeaways:- Chemical reactions follow predictable rules.
- Elements combine in specific ratios.
- Mass remains constant during reactions.
Simplifying Complex Chemistry
Understanding these laws provides a solid foundation for exploring chemistry. By recognizing patterns and relationships, you'll better grasp chemical reactions and compound formation.
"This Content Sponsored by Genreviews.Online
Genreviews.online is One of the Review Portal Site
Website Link: https://genreviews.online/
Sponsor Content: #genreviews.online, #genreviews, #productreviews, #bestreviews, #reviewportal"




Comments
Post a Comment